Electroplating is an electrochemical process in which the shape of a product is recreated by the deposition of metal on it. The method of electroforming involves coating metal with non-metallic surfaces.
Technology application
Electroplating is often applied to various elegant objects (jewelry, orders and medals, coins, shells, flower pots, sculptures, portraits, etc.). Most often, copper is used in electroforming. However, other metals may be used, including nickel, chromium, steel, silver.
Subject to all technological requirements, it is possible to distinguish the copied object from the original only by the barrier layer or by removing the original. And all the work is quite possible to do it yourself at home.
to contents ↑Note! The coating of the product to be copied must be electrically conductive. If the material is devoid of this property, bronze or graphite is applied to it.
Form creation
From the product that we will copy, we take the print. To do this, you will need some fusible metal, plasticine, gypsum or wax. If we use metal, we process the copied object with soap and put it in a cardboard box. Next, fill in the low-melting alloy.
When the casting is completed, we take out the product and the resulting mold is first subjected to degreasing, and then copper plating in the electrolyte. To avoid metal deposits on those sides where there is no print, we melt the metal in boiling water to obtain a matrix. Fill the form with plaster. The output is a copy.
To create a matrix, you need such a composition:
- wax - 20 parts;
- paraffin - 3 parts;
- graphite - 1 part.
If the form is created from a dielectric material, we apply an electrically conductive coating to its surface. The conductor layer is applied either by metal reduction, or by mechanical means, involving the application of scaly graphite using a brush.
Even before the start of surface machining, we grind graphite in a mortar, sift it through a sieve. The best adhesion of graphite is observed with plasticine. Plaster, wooden, glass and plastic forms, as well as papier-mâché, are most effectively treated with a solution of gasoline and wax. When the surface has not dried out yet, we apply graphite dust to it, and we blow off the adhering substance with a directed stream of air.
The plating is easy to separate from the matrix. If the form is metallic, we create an oxide or sulfide conductive film on the surface. For example, on silver it will be chloride, on lead - sulfide. The film will help to easily separate the form from the coating. In the case of copper, silver, and lead, we coat the surface with a 1% sodium sulfide solution to form insoluble sulfides.
to contents ↑Materials and equipment
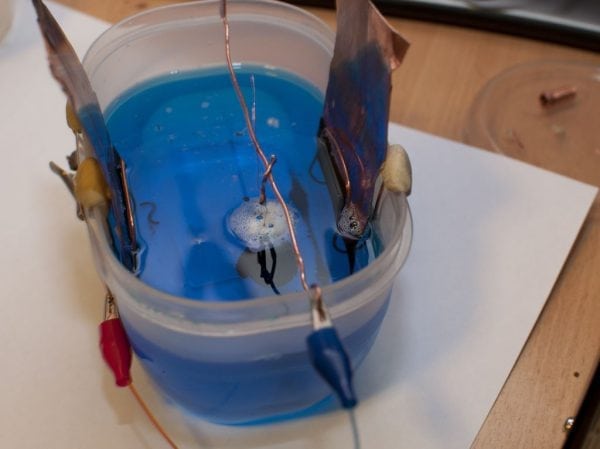
When the form is ready, put it in a galvanic bath connected to an electric current (to prevent the separation of the separating film). First, we conduct the coating of the conductive copper layer under conditions of low current density.
We will need the following composition:
- copper sulfate - 150-200 grams;
- sulfuric acid - 7-15 grams;
- ethyl alcohol - 30-50 milliliters;
- water - 1 liter.
The working temperature in the electrolyte bath is 18-25 degrees Celsius. Current density - from 1 to 2 Amperes per square decimeter. Alcohol will be needed to improve the wettability of the coating. As a constant current source, you can use a charger for car batteries. We also need an ammeter with the ability to measure current from 0 to 3 or 5 amperes. Usually an ammeter is already available for charging.
The nichrome wire will serve as a rheostat. We wrap it on any ceramic plate. The spiral from the electric heater will fit perfectly.
As a bath, any plastic container with a volume of 2 to 50 liters is suitable, depending on the needs. We use a copper plate as an anode.
Note! The area of the anode should be approximately equal to the area of the workpieces.
To create a conductive layer for the product, add a few drops of varnish to the bronze powder. Colorless nitrol varnish is recommended. The varnish needs to be made more liquid, so we dilute it with acetone to the consistency of a liquid paint composition.
to contents ↑Manufacturing process
We take about a 20-centimeter section of a multicore cable and remove the wire from it. We protect the insulation on both sides of the wire, bend one end of it at an angle of 90 degrees and glue it to the plastic part with instant glue. Moreover, the BF glue will not work, since it will dissolve bronze paint.
When the objects are dry, we degrease them using household chemicals (for example, washing powder). Next, we wash the product in running water or treat it with acetone.
Details are firmly fixed to the wire. Now they can be dipped one by one in a pre-prepared bronze paint or applied with a brush. The entire surface should be uniformly painted. It is recommended to use an insulated wire from the cable, otherwise copper will fall on the bare wire, which will lead to additional consumption of the anode.
After drying the surface for an hour, twist the dried ends of the wires together. Parts must not be in contact with each other. Next, we connect the products to the positive contact and immerse them in the bath. A few seconds after the dive, the process of copper-plating, noticeable with the naked eye, will begin.
The thickness of the copper coating may vary depending on the circumstances, but for small items it will be approximately 0.05 millimeters. In the bath, the parts are kept for 15 hours. The current is regulated by moving the contact along the nichrome rheostat within 0.8-1.0 amperes. After copper plating, increase the current to 2 Amps. When the exposure time of the parts expires, we rinse objects in running water, dry them, and cut off the wire. We clean the wire and prepare it for the next procedure.
Metallization completed. Next, we take sulfuric ointment (available at the pharmacy), apply it to the surface and carry the item on the fire of a gas stove. In this case, copper will immediately darken.
The next step is polishing. For this, a motor equipped with a round metal brush is useful. This work requires a certain skill. As a result, we should get a surface that looks like blackened bronze with individual shiny areas. If it was not immediately possible to achieve the desired result, again we apply sulfuric ointment, heat the product over the fire and polish it.
For those who doubt the effectiveness of the procedure described above, we suggest making a test. To do this, you need a capacity for the electrolyte, where you need to lower a little copper. Paint one part from the spray gun with 2-3 layers in bronze color. Next, you need to connect to the battery without using a rheostat. An adapter from the player is also suitable.
to contents ↑
Other metals
In addition to copper, other metals, including gold or silver, can also be applied to a non-metallic surface. Silver electroplating can be carried out in one of two ways: chemical or electrochemical. Chemical silvering is done by immersing the product in a boiled solution with silver. The electrochemical process gives a more reliable result, since the coating is more durable as a result of exposure to electric current. Silver electroplating is widely used in jewelry manufacturing.
So, electroplating at home is quite possible. The process is quite time-consuming and requires certain skills, but the end result is worth it.









To dig a hole in the Earth with a wide, polyethylene film, lay it out from the inside, monolithic, the ends of which are brought out of the hole., And lay them there with bricks, and on top of this hole cover the second with the same film, the ends of which are also surrounded with bricks around this hole, and inside this a pit dug and draped over its bottom and sides with a plastic film, pouring there a solution of acid and copper sulphate, conduct electroplating of large structures in their size, such as, for example, large parabolic mirrors, the body of an electric vehicle, etc. !!! Wish you happiness!!!
in approximately the same way a quadriga was made over the pediment of the Bolshoi Theater)))