Anodizing aluminum (anodic oxidation) is the process by which an oxide coating forms on the surface of a metal. The main task of the oxide coating is to protect the aluminum surface from oxidation arising from the interaction of this metal with air. Anodizing is intended not to destroy the film formed during oxidation (it performs a protective function), but to make it more durable. In this regard, anodizing is similar to a method such as oxidation burnishing.
- Anodizing technology
- Preparatory process
- Chemical treatment
- Fastening
- Other methods of anodizing
- Home Anodizing
- Solution preparation
- Anodizing

Anodic oxidation technology is used to strengthen not only aluminum and its alloys, but also other metals. For example, oxide coatings are used to protect titanium and magnesium.
In addition to strengthening the surface layer, anodizing has the following objectives:
- smoothing of various surface defects (chips, scratches, etc.);
- improving the adhesive qualities of the material (paint adheres much better to an oxide film than to bare metal);
- improving the appearance of the metal;
- giving the metal various decorative effects (for example, you can create an imitation of gold, silver, pearls).
Anodizing technology
The anodizing process can be divided into three parts:
- preparatory process;
- chemical treatment;
- fastening.
Preparatory process
At this stage, the aluminum profile is subjected to mechanical and electrochemical processing. Machining refers to the cleaning of metal, its grinding and degreasing. Next, the product is first placed in an alkaline solution for etching, and then transferred to acid for clarification. The preparation is completed by flushing the surface. Moreover, washing is carried out several times to completely remove acidic substances from the metal.
Chemical treatment
Chemical oxidation of aluminum is the processing of metal in an electrolyte. Solutions of various acids (sulfuric, chromic, oxalic, sulfosalicylic) are used as electrolytes. Sometimes salt or organic acid is added to the solutions.
The most common electrolyte is sulfuric acid. And yet, this electrolyte is not used for processing products of complex shape, on which there are small holes or gaps. In such cases, chromic acid is preferred. But oxalic acid can significantly improve multi-colored insulation coatings.
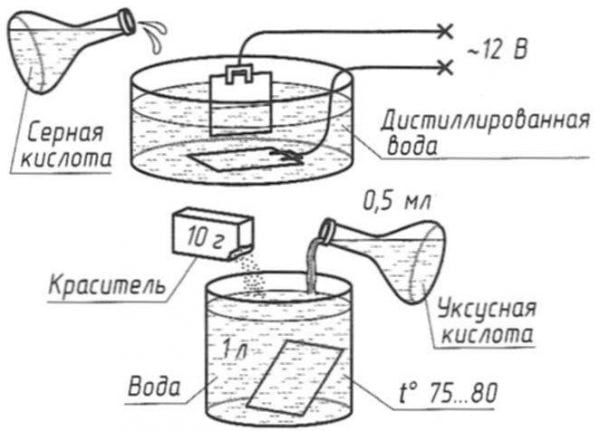
The quality of the process depends on several components, including concentration, temperature and current density. High temperatures accelerate anodizing. Moreover, the film is formed soft and highly porous. If a hard coating is required, a lower temperature is applied.
Chemical oxidation of aluminum can be carried out at temperatures from zero to plus 50 degrees Celsius. The current density can vary from 1 to 3 amperes per square decimeter. The electrolyte concentration may be in the range of 10-20%.
to contents ↑Fastening
After oxidation, the metal looks like a porous surface (even when using the cold mode). In order for the surface to be strong enough, these pores must be closed. This is done in one of three ways:
- dipping the product in hot fresh water;
- steam treatment;
- the placement of metal in the so-called "cold solution".
Note! If the product will be painted, the fixing process is not needed, since the paint material will naturally fill the existing pores.
There are three types of aluminum oxidation equipment:
- main (baths);
- serving (job security);
- auxiliary (supply of products to the bath, preparation, storage, etc.).
Other methods of anodizing
In addition to the classical method described above, solid, microarc and color anodizing can also be used. These metal processing methods will be briefly described below.
The task of solid anodizing is to obtain a particularly durable microfilm. The technique is widely used in aircraft manufacturing, automotive and construction. A feature of the technology is that not one, but several electrolytes are involved at once. For example, oxalic, sulfuric, citric, tartaric, and boric acids can be used in a single process. During anodization, the current density gradually increases, and due to structural changes in the cells, the film acquires increased strength.
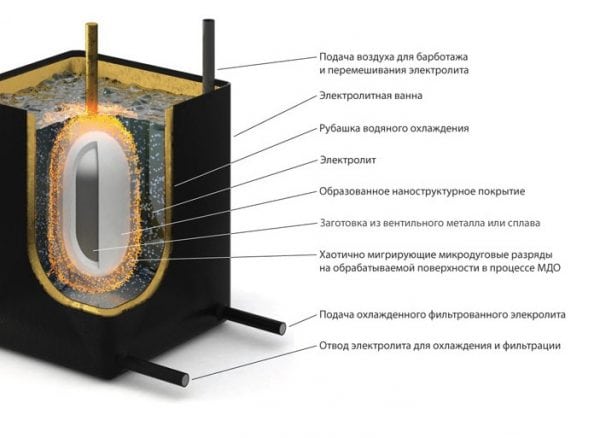
Microarc oxidation is an electrochemical process in which the surface of aluminum is oxidized, and at the same time, electric charges occur between the anode and electrolyte. The technique allows to obtain particularly high-quality coatings with a high level of wear resistance and adhesion.
Another way of anodizing is color. As the name implies, the main task of the process is to change the color of the part.
There are four ways to color anodize:
- Adsorption staining. It is carried out by immersing the product in an electrolyte bath. It is also possible to immerse the part in a solution with a coloring matter heated to a predetermined temperature.
- Electrolytic staining (another name is black anodizing). A colorless film is first obtained, and then the metal is dipped in acidic saline. At the output, the color of the product can vary from black to a faint bronze hue. Black tones of aluminum are especially in demand in the construction industry.
- Interference staining. The technology is similar to electrolytic staining, but due to the creation of a special reflective layer, color shades are much more diverse.
- Integral staining. The technology is mixing electrolyte with organic salts.
to contents ↑
Home Anodizing
Self-anodizing is almost always carried out by the cold method. Most companies providing similar services adhere to the same technology. The cold technique is called due to the fact that in the process of creating a film there is no need for high temperatures: the working temperature range ranges between -10 and +10 degrees Celsius.
Advantages of cold anodizing:
- The surface layer is quite thick due to the fact that the growth and dissolution of the oxide film from its outer and inner sides are different.
- The film comes out very durable.
- Processed metal is highly resistant to corrosion.
The only drawback of the technique is the difficulty of further painting the metal with materials based on organic matter. However, the metal, regardless of its characteristics, in any case, gets a natural color. Color can vary from olive to black or grayish.
For the work you will need the following:
- bathtubs (aluminum containers for anodizing, as well as a pair of glass or plastic - for the manufacture of solutions);
- aluminum connecting wires;
- 12 volt voltage source;
- rheostat;
- ammeter.
Solution preparation
As mentioned above, the main electrolyte for anodizing is sulfuric acid. However, outside the manufacturing facility, the use of such an electrolyte is dangerous. Therefore, at home, soda is usually used.
Solution preparation:
- We prepare 2 solutions - soda and hydrochloric. The components are poured into containers with distilled warm water in a ratio of 1 to 9.
- Mix the solution well and let it brew.
- Drain the solution into another container so that soda precipitate does not get there. The result of anodization depends to a large extent on the purity of the solution.
Anodizing
First of all, you need to prepare the part. The task of the preparatory process is to clean, grind and degrease the surface before anodizing. If the product does not remove visible defects, the resulting film will not be able to hide them, since its thickness does not exceed 1/20 mm. Just before anodizing, mix both solutions in one bowl.
The anodizing tank must be large enough so that the part can be completely immersed in it. In addition, the part must be fixed so as not to touch the bottom of the dishes. To do this, you can use the rack or any other option - at your own discretion. You also need to carefully consider the issue of fixing the part, since after anodizing in the places of fixation there will be traces.
Power is supplied for at least 30 minutes. The need to complete anodizing is indicated by a change in the color of the part. When the part is ready, turn off the voltage, and remove the metal from the bath.
After removal, thoroughly rinse the workpiece. To ensure a high-quality result, put the metal in a manganese solution for 15 minutes. Then we rinse the part first in warm and then in cold water. Next, dry the metal. If the technology is not broken, the product will acquire a light gray tone. The work done qualitatively is indicated by a uniform surface color, the absence of streaks and spots.
The final stage of anodizing is film fixing. It is necessary to close the microscopic pores present in the film coating. To do this, put the metal in a container with distilled water and boil for half an hour.
If desired, a metal surface can also be painted or varnished. The paint layer is applied by immersion.
So, anodizing aluminum can be carried out in various ways. However, only cold metal treatment with soda and saline solutions is available at home. It is also worth noting that, subject to technological requirements, regardless of the type of solution, there is no significant difference in the quality of the surfaces obtained.